Purified gelatins support safe and effective delivery of drug and cell therapies
Drug delivery faces many challenges, all of which depend on the type of drug, method of delivery and bioavailability of the vehicle in which it is delivered. Gelatin's unique properties and long-standing history of safe in-body use in various medical applications, since the first successful gelatin-based plasma substitute in 1915, have made it a widely utilized drug delivery carrier.
Rousselot – your expert partner for biodelivery solutions
Our experienced team will recommend an optimal medical grade gelatin or modified gelatin for your specific drug delivery application, customizing a tailored solution to support drug development from formulation to the most advanced delivery, with high purity, quality and consistency.
Applications of X-Pure® gelatin in bio delivery
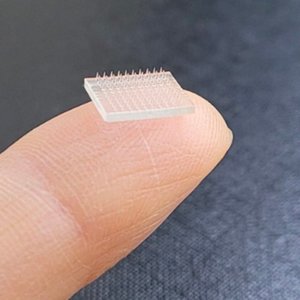
Customizable gelatin for microneedle applications
X-Pure® supports uniform patch fabrication and scalable production.
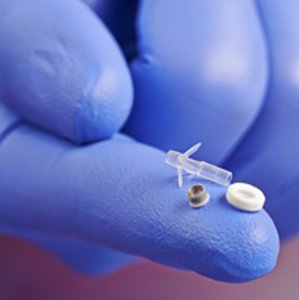
Purified gelatin for drug delivery implants (in body)
X-Pure® can be tailored to achieve the optimal level of controlled or sustained release, texture, dose & desired pharmacokinetics parameters.
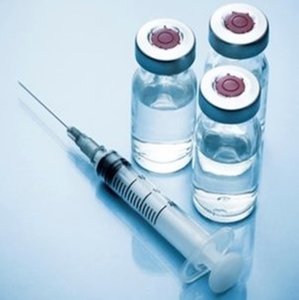
Purified gelatins for vaccines
Rousselot hydrolyzed gelatins help to stabilize vaccines while minimizing the risk of endotoxin-induced immune responses.
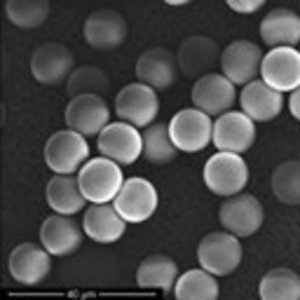
Micro- and submicro- particle applications
X-Pure® gelatins can be shaped into different structures to support specific, targeted and controlled release.
Why is gelatin an ideal delivery vehicle for biomolecules?
- Excellent biodegradability: Gelatin is a protein derived from collagen. It is naturally degraded by enzymes in the body (metalloproteinases).
- No immune response: Gelatin is safe and does not stimulate an immune response in the body.
- Compatible with multiple APIs: Gelatin protects the cell membrane and stabilises proteins. It is therefore compatible with many different APIs, RNA, DNA, cells and biologics.
- Versatility: Gelatin can be shaped into different structures to support specific, targeted and controlled release for a wide range of delivery methods, including large and small molecules encapsulated in micro and nano-spheres, gel films, colloidal injectables, ocular inserts, eyedrops, microneedles and implants. 1
- Tunable release: By forming complexes with different drugs and tuning parameters, including degree of crosslinking and molecular weight, it is possible to optimize gelatin’s degradation and drug delivery kinetics.
How can Rousselot help you optimize drug or cell delivery?
Our experienced team can help you maximize the potential of gelatin-based biomaterials and design highly accurate and precise delivery systems.
1. Ultra-low endotoxins to support patient safety

Endotoxins can cause significant adverse effects in drug delivery applications.2 To overcome this problem, Rousselot’s X-Pure® range is ultra-purified and has been developed to ensure maximum quality and safety.3
2. Customizable for precise and efficient drug or cell delivery

Rousselot can help you select the gelatin with the right mechanical properties to ensure optimal spatio-temporal control. With tunable properties including consistency, adhesiveness and biodegradability, X-Pure® gelatins enable you to achieve precise timing and targeted short term or sustained release of drugs or cells within the body.
Modified gelatin designed for drug delivery: X-Pure® gelatins can be modified (or functionalized) to bind, carry, and deliver drugs to targeted sites in the body, enhancing their therapeutic efficacy and reducing potential side effects.
Gelatin with tunable mechanical properties for cell delivery: X-Pure® gelatins have customizable mechanical properties to recreate an environment that mimics the cell’s natural extracellular matrix for successful cell culture. By tuning the molecular weight and level of crosslinking, X-Pure's biodegradability can be tuned to meet your delivery targets.
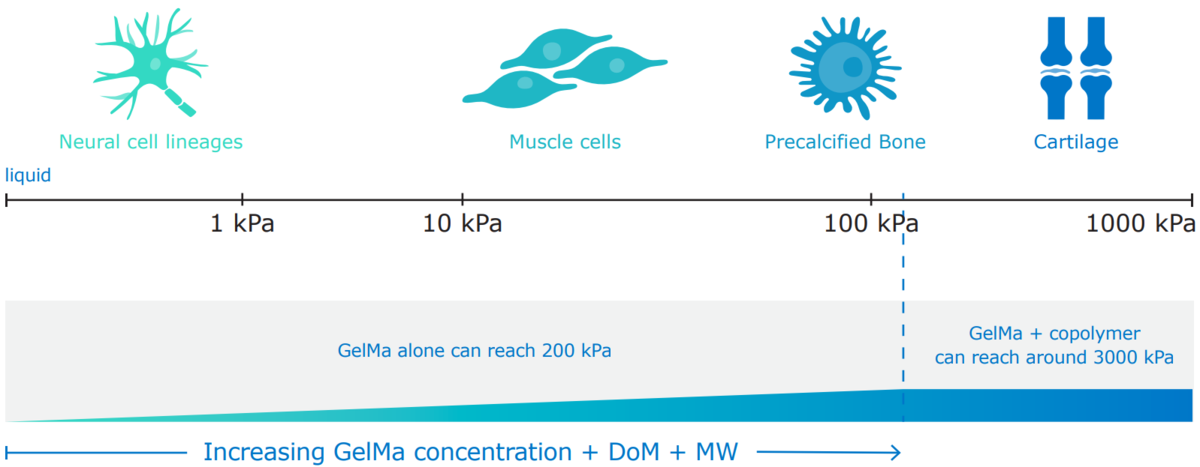
Figure 1: Different cells require different environments.
3. Compliance

All X-Pure® and Quali-Pure® gelatins are compliant with the requirements of the major pharmacopoeia and support regulatory compliance with the EU Medical Device Regulation (MDR) 2017/745 and US FDA guidance for medical devices. Our biomaterials come with full traceability, readily available documentation and validated viral inactivation as also required by ISO 22442, helping to save time when reaching the clinic.
References:
- Nikkhah, Medhi, Mohsen Akbari, Arghya Paul, Adnan Memic, Alireza Dolatshahi- Pirouz, and Ali Khademhosseini. 2016. Gelatin-based biomaterials for tissue engineering and stem cell bioengineering. In Biomaterials from Nature for Advanced Devices and Therapies, by Nuno M Never and Rui I Reis, 37-62. John Wiley & Sons, Inc.
- Vallhov, H, J Qin, SM Johansson, N Ahlborg, MA Muhammed, A Scheynius, and S Gabrielsson. 2006. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett 6 (8): 1682-6. doi:10.1021/nl060860z.
- Olijve, J and Vanhoecke, B. 2019. Low Endotoxin Gelatins and Collagens For (Bio)Medical Applications.
