
Gelatin is a versatile and reliable biomaterial derived from native collagen, a natural protein found in the extracellular matrix of animals. Due to its unique properties – including cell recognition sites, excellent biocompatibility, low antigenicity and tunable biodegradability – gelatin provides a wide range of exciting opportunities in regenerative medicine. The simplicity of its molecular structure means it can be modified to improve its biological and functional properties, opening up a world of possibilities for biomaterials that can support innovations in tissue engineering, cellular therapy, advanced drug delivery and more.
What makes gelatin so special as a biomaterial?

The field of biomaterials is progressing rapidly due to recent advancements in chemistry, mechanobiology, cutting-edge technologies, novel cell sources, avant-garde drug delivery platforms, and bio fabrication processes. Gelatin is a particularly fruitful biomaterial, thanks to its innate biological characteristics and tunable physical properties, which can be harnessed to create additional functional capabilities compared to collagen. This natural polymer is commonly used for medical applications due to its versatility, reliability and biocompatibility in physiological environments. Among the different natural sources of biomaterials, gelatin is the most readily available and cost-effective compared to other extracellular matrix (ECM) proteins.
Gelatin is normally derived from Type I collagen, the most abundant collagen found in connective tissues including skin, tendon and bone tissue. It is obtained through partial hydrolysis, which irreversibly denatures collagen’s triple helical structure. Gelatin has several chemical properties that make it well-suited to cell culture applications. For example, it contains integrin binding peptides that facilitate cell adhesion. The main receptor-recognition motif of gelatin is the tripeptide Arg-Gly-Asp (RGD), which provides a biologically active 3D microenvironment that mediates a plethora of cellular mechanisms, such as cell adhesion, growth, differentiation, migration, signaling and survival(2, 3). Gelatin also contains metalloproteinases (MMP)-sensitive peptides which play an important role in tissue regeneration (4).
Besides these cell-stimulatory qualities, gelatin has other beneficial attributes which make it suitable for a wide range of biomedical applications beyond cell culture. For instance, gelatin is an ideal delivery vehicle for biomolecules due to its ability to create poly-ionic complexes with charged therapeutic compounds(5), such as, nucleotides, proteins, growth factors and polysaccharides. Gelatin’s thermo-responsive character, which allows it to undergo a reversible phase transition from a liquid to a gel, means it can slowly release biomolecules out to the environment over time, thereby enhancing therapeutic effects(4).
The potential of modified gelatins for biomedical applications

The relatively low melting temperature of gelatin (31.7–34.2 ◦C) can restrict its use under physiological conditions(6). However, by adding other components to stabilize the gel, such as methacrylate anhydride (MA), and performing photo-induced polymerization (chemical crosslinking), it is possible to obtain a hydrogel with a higher melting point than that of gelatin alone(7). By controlling the production parameters (i.e., hydrogel concentration, degree of functionalization, UV intensity, and additive supplementation), it is possible to produce a range of different GelMAs tailored to different cell types. Customizing the microenvironment in this way can help to promote cell growth, differentiation, migration and function. Another way to modify gelatin is through physical crosslinking, but this method has limited use in complex microenvironments because it leads to irregular network structure, poor mechanical strength and reversibility(8).
The mechanical properties of GelMA and gelatin can be customized in many different ways to suit an ever-expanding list of biomedical applications. This can be as simple as varying the gel concentration, or as complex as coupling numerous types of natural, inorganic or organic polymers, natural proteins or polysaccharides to provide additional functionalized moieties(9). The benefits of gelatin‐composites can also be applied to other types of biomaterial platforms, such as 2D coatings.
Although GelMA and gelatin are highly versatile and provide exceptional conditions for cells, they cannot provide the rigid structure needed for bone cell development or the electromechanical properties required to grow skeletal muscle, cardiac and neural cells. These drawbacks can be overcome by modifying hydrogels with nanomaterials to produce hybrid biomaterials that are specifically designed to mimic the extracellular environment of certain cells and stimulate their functions. For instance, adding carbon-based materials can increase the strength and electrical conductivity of the biomaterial while retaining similar degradability, porosity and cell dispersion properties to GelMA(10).
Biomaterials that can take you from research to patient
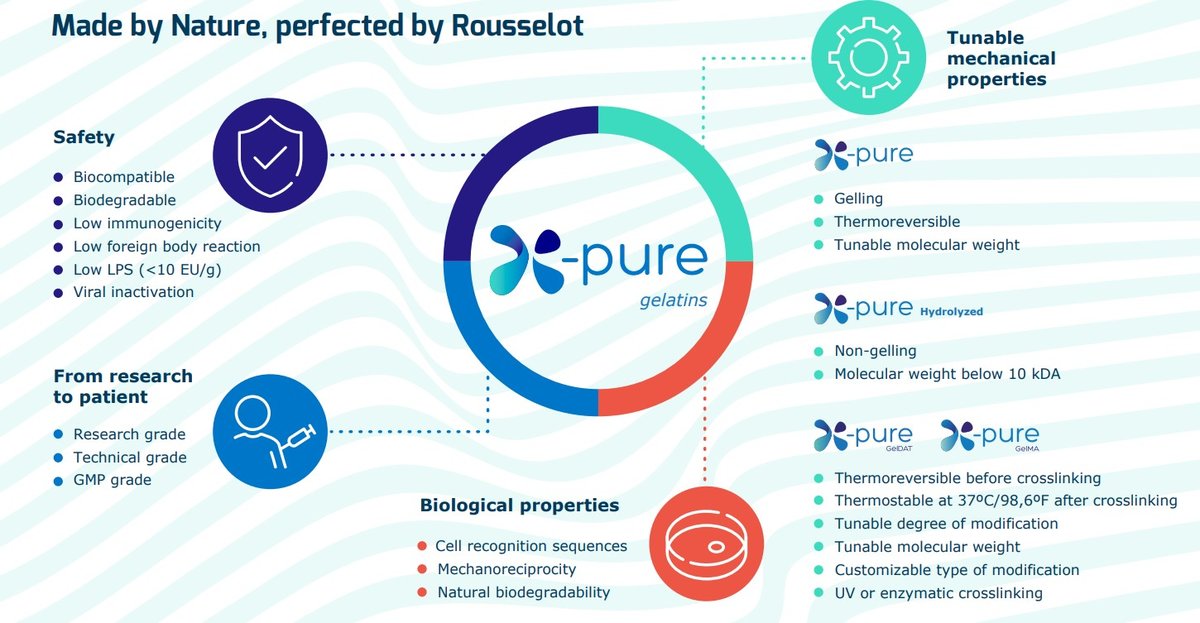
Collagen-based biomaterials offer enormous potential for regenerative medicine applications due to their biological and functional attributes, including having similar mechanical and adhesive properties to the natural ECM. However, some scientists are wary of these natural products due to batch variability, short degradation periods and difficulties in purification and quality control. Fortunately, biomedical companies, such as Rousselot, are making steadfast advancements in the production of high-quality, reliable biomaterials to help overcome these drawbacks. By integrating expertise across different fields such as manufacturing technology, material science and biology, Rousselot has developed a range of commercially available collagen-based products such as X-Pure® (Figure 1) that can be used throughout the entire clinical translation pathway, from research to patient.
Figure 1
References
- Gorgieva, Selestina & Kokol, Vanja. (2011). Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. DOI: 10.5772/24118
- Davidenko N, Schuster CF, Bax DV, Farndale RW, Hamaia S, Best SM, Cameron RE. Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry. J Mater Sci Mater Med. 2016 Oct;27(10):148. DOI: 10.1007/s10856-016-5763-9.
- Zaman MH. Understanding the molecular basis for differential binding of integrins to collagen and gelatin. Biophys J. 2007 Jan 15;92(2):L17-9. DOI: 10.1529/biophysj.106.097519.
- Echave, M.C.;Hernáez-Moya, R.;Iturriaga, L.;Pedraz, J.L.;Lakshminarayanan, R.;Dolatshahi-Pirouz, A.;Taebnia, N.;Orive, G. Recent advances in gelatin-based therapeutics. Expert Opin Biol Ther. 2019, 19, 773-779, DOI: 10.1080/14712598.2019.1610383
- Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Current Opinion in Cell Biology. 2006 Oct;18(5):463-471. DOI: 10.1016/j.ceb.2006.08.009
- Mad-Ali S, Benjakul S, Prodpran T, Maqsood S. Characteristics and gelling properties of gelatin from goat skin as affected by drying methods. J Food Sci Technol. 2017;54(6):1646-1654. doi:10.1007/s13197-017-2597-5
- Bupphathong S, Quiroz C, Huang W, Chung PF, Tao HY, Lin CH. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications-A Review on Material Modifications. Pharmaceuticals (Basel). 2022 Jan 29;15(2):171. DOI: 10.3390/ph15020171
- Liu J, Su C, Chen Y, Tian S, Lu C, Huang W, Lv Q. Current Understanding of the Applications of Photocrosslinked Hydrogels in Biomedical Engineering. Gels. 2022 Apr 1;8(4):216. doi: 10.3390/gels8040216
- Bello, A.B.;Kim, D.;Kim, D.;Park, H.;Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng Part B Rev. 2020, 26, 164-180, DOI: 10.1089/ten.TEB.2019.0256
- Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015 Dec;73:254-71. DOI: 10.1016/j.biomaterials.2015.08.045
