Our GelMA expert Dr. Jeff Daelman will share how Rousselot Biomedical re-engineered the gelatin modification process and will talk about process, consistency, purity, and safety, in his Company Presentation.
Registered delegates can watch this presentation from today
Highlights include:
- How co-developing gelatin biomaterials has delivered optimal biomedical biomaterial for use in clinical settings
- Why scalability matters and how to avoid having to re-validate your excipient
- Why selecting a gelatin with the end-use in mind helps save time and money
- Regulatory limits – The levels of purity required to meet endotoxin limits set by regulators such as the FDA.
Find out how Rousselot Biomedical unlocks the next level in the development of gelatin-based biomedical products, including a close look at X-Pure GelMA and the recently launched X-Pure GelDAT.
X-Pure GelMA is the world’s first GMP ready gelatin methacryloyl (GelMA) for preclinical and clinical applications, ideal for 3D bioprinting and tissue engineering owing to its guaranteed ultra-low impurity levels and tunable mechanical properties.
X-Pure GelDAT (Gelatin Desaminotyrosine ) is the world’s first ever purified and phenol-functionalized gelatin. X-Pure GelDAT hydrogels are ideal for research and preclinical development in regenerative medicine, drug release and complex wound dressings due to their ability to crosslink with tissues, their strong adhesive properties and their purity.
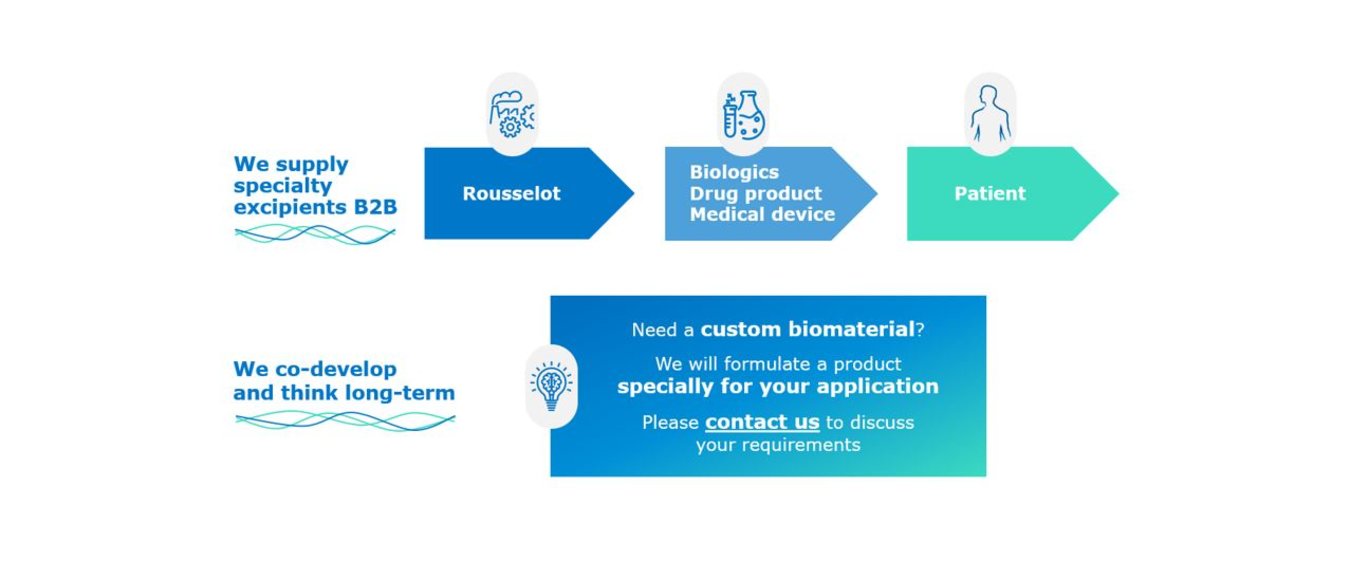
Co-development is key to creating the biomaterial you need for your biomedical application.
Meet our partnering team: Dr. Jeff Daelman, Jos Olijve and Jeroen Geeraerts