Watch our videos on X-Pure
Endotoxins & cellular activity
In biomedical R&D and clinical applications, endotoxins can trigger a strong fatal immune response. X-Pure GelMA minimizes the risk of prolonged or sustained inflamation caused by endotoxin exposure. Jos Olijve, Rousselot Scientific Support Manager, presents a test that was done to know how the level of endotoxins in GelMA influences in-vitro culturing applications.
No more batch lottery when making a new GelMA
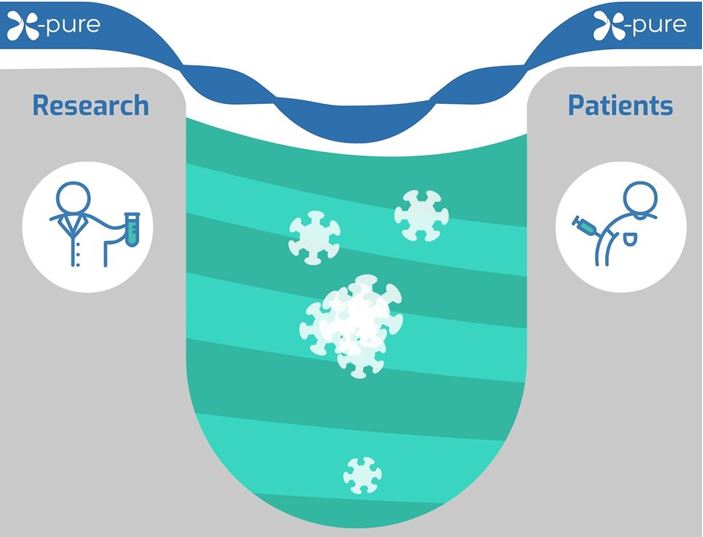
X-Pure - Bridging the valley of death
For ultra-low endotoxin biomaterial, choose X-Pure
Endotoxins can negatively impact cell viability, proliferation, functionality, and differentiation. It’s therefore important to control the endotoxin levels during cell culturing. The use of gelatins with unknown endotoxin levels in in vitro research can contribute to irreproducible results and can delay laboratory to clinic translation.
The X-Pure range offers gelatins and collagens with extremely low endotoxin levels, delivering consistent quality and traceability to the source for a wide range of applications. Watch our video to learn more about X-Pure and why endotoxins are the unwanted contaminants of biomaterials.